2 See answers. So it was better to choose Carbon-12 isotope as the standard.
Global Monitoring Laboratory Carbon Cycle Greenhouse Gases
By chance defining the atomic mass as 116th of the mass of a mole of oxygen comprising a natural mix of 16 O 17 O and 18 O is very close to a standard defining the atomic mass as 112 the mass of a.

. The carbon-based standard represented a nice compromise. Why carbon 12 isotope is taken as standard for measurement of atomic mass. The standard to be chosen should not be very small or very large compared to the atoms that are to be measured.
There are several actual reasons why Carbon-12 is taken as the standard of atomic mass measurement. This is due to two factors. Carbon-12 is the standard while measuring the atomic masses.
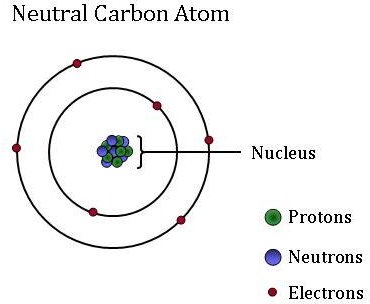
Carbon-12 is the most abundant of the two stable isotopes of carbon amounting to 9893 of element carbon on Earth. Why carbon -12 isotope is taken standard in AMUcarbon12amuatomicmassunittopics to be covered why carbon 12 is taken as standardcarbon 12atomic mass un. The history of atomic mass and the mole the two are quite interconnected goes back to the early 19th century to.
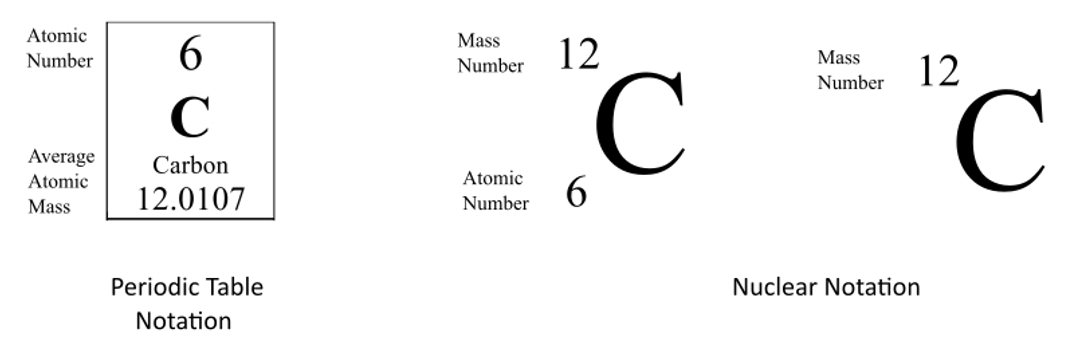
Carbon 12 was chosen because the chemical atomic weights based on C12 are almost identical to the chemical atomic weights based on the natural mix of oxygen. Nothing special about it. The Carbon-12 atom having an atomic mass of exactly 12u is just a consequence of the definition of the atomic mass unit.
As is the case elsewhere in metrology the answer is tied up in history measurability practicality repeatability past misconceptions and consistency despite those past misconceptions. Carbon-12 is taken as the standard reference of atomic mass universally as It was chosen because of its coherence to Avogadros Principle its stability and abundance and basically to stop everybody from fighting as earlier Hydrogen-1 and Oxygen-16 were also used for measuring atomic mass which were less accurate. Why was Carbon-12 chosen for the atomic mass unit.
C-12 is the most abundant isotope of carbon which constitutes 989 of available isotopes of carbon. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured thus its atomic mass is exactly 12 daltons by definition. 1 the different mass of neutrons and protons acting to change the total mass in nuclides with protonneutron ratios other than the 11 ratio of carbon-12.
Because no other nuclides other than carbon-12 have exactly whole-number masses in this scale. - 13847202 DynamicPlayer DynamicPlayer 30112019 Physics Secondary School Why carbon 12 isotope is taken as standard for measurement of atomic mass. And 2 an exact whole-number.
Its abundance is due to the triple-alpha process by which it is created in stars.

Carbon Facts About An Element That Is A Key Ingredient For Life On Earth Live Science

States Of Matter Https Youtu Be Wd9zih3nbxm Relative Atomic Mass Carbon 12 Chemistry Lecture

0 Comments